Active immunotherapy for colorectal cancer
Colorectal cancer is the third most common cancer worldwide. Surgical resection of early-stage localized disease is the only available treatment modality, as an advanced colon cancer is usually resistant to cytotoxic therapy, including chemotherapy and radiotherapy. On the other hand, evidence has accumulated that immune-based approaches could significantly ameliorate the course of disease.
We performed a case-control study to evaluate the overall 2-year survival rate in 37 vaccine-treated colorectal cancer patients (clinical characteristics are presented in Table 3). All patients from the treatment group had a confirmed disease diagnosis, and the control group was composed retrospectively to combine patients who received conventional therapy previously, with matched prognostic and clinical characteristics of patients from control and treatment groups. Throughout the follow-up period, patients from the treatment group received no systemic therapy, other than immunotherapy.
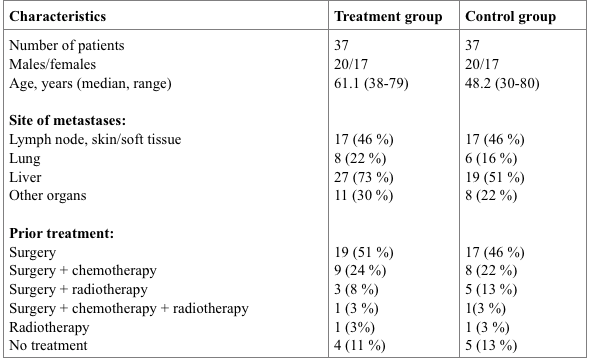
Table 3. Clinical characteristics of patients recruited in the case-control study.
As shown in Figure 5, the median survival rate in XPV-treated patients was significantly longer (P < 0.05), compared to that in control patients (17 vs. 7 months, respectively). Overall 2-year survival rates in XPV-treated and control groups were 27% (10 patients) and 3% (1 patient), respectively. Clinical effects of various degrees (complete response, partial response and disease stabilization) with duration of ≥6 months was observed in 23/37 (62%) patients from the treatment group.
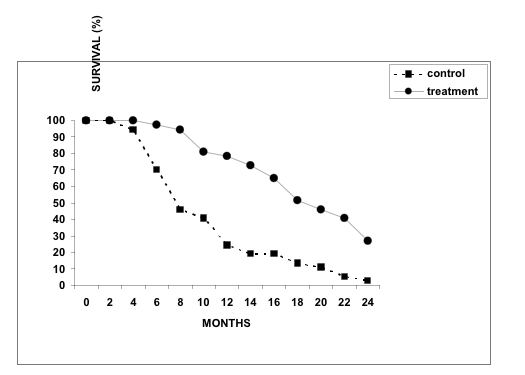
Figure 5. Survival rates in patients from control (n=37) and XPV-treated (n=37) groups.
Importantly, this clinical study included patients with very advanced disease. We envisage that XPV immunotherapy is most effective when initiated before or immediately after surgical resection of the primary tumor and its regional metastases.
Examples:
Case 1. A 51-year-old female patient was admitted to a surgery department with acute bowel obstruction. During laparotomy, tumor conglomerates were found in the sigmoid colon, in the retroperitoneal space and in the left uterine appendages. In addition, a mesocolon abscess was detected. Cytoreductive surgery with a colostomy was performed. A mucus-producing adenocarcinoma was diagnosed by histological analysis. A vaccine therapy course was initiated after patient’s discharge from the hospital, which was well tolerated. Secondary liver tumor lesions (up to 25 mm) were detected 10 months after the immunotherapy onset. Ultrasonography confirmed a 34×27 mm solid mass in the left retroperitoneal space. Nevertheless, we continued an immunotherapy treatment of the patient. One year later, ultrasonography and computerized tomography (CT) revealed no signs of metastatic lesions in the liver, and anastomosis/colostomy closure was performed. A final 3-year follow-up examination after vaccine therapy found the patient in a good condition without serious complains.
Case 2. A 46-year-old male patient underwent surgery for colon cancer. A low-grade differentiated adenocarcinoma was diagnosed by histological examination. A focal 15 mm liver lesion was found by ultrasonography and CT. A vaccine therapy course was initiated, and no secondary liver lesions were detected by ultrasonography 1.5 years after immunotherapy treatment in June 2001. A 4-year follow-up examination showed that the patient was in good condition without any signs of disease.
